Realization of ultra-fine mass spectrometry imaging method using pico-liquid and its application to biomedical field
Mass spectrometry imaging is characterized by its ability to visualize the distribution of chemical components in biological tissues, and is expected to be applied to the investigation and diagnosis of diseases on the basis of scientific evidence in the future.
In this project, we aim to realize an innovative ultra-fine mass spectrometry imaging method based on the extraction-ionization method (t-SPESI), which has been developed by the project leader, using precise fluid control of a micro-volume liquid.
We will also promote interdisciplinary collaborative research to obtain knowledge that will lead to the identification and understanding of diseases by capturing changes of various components in diseased tissues and cells as multidimensional chemical distribution information.
FEATURE
Promoting research and development of unique technology to extract and ionize sample components in the micrometer range using picoliters of solvent.
Precise control of picoliter solvents based on physical understanding through instrument development and analysis of measurement data.
Collaborating with researchers in the fields of biochemistry and medicine, we aim to understand the state of health using information on the distribution of various components of biological specimens.
RESULTS
Research progress
Development of extraction-ionization method utilizing flow of picoliter solvent
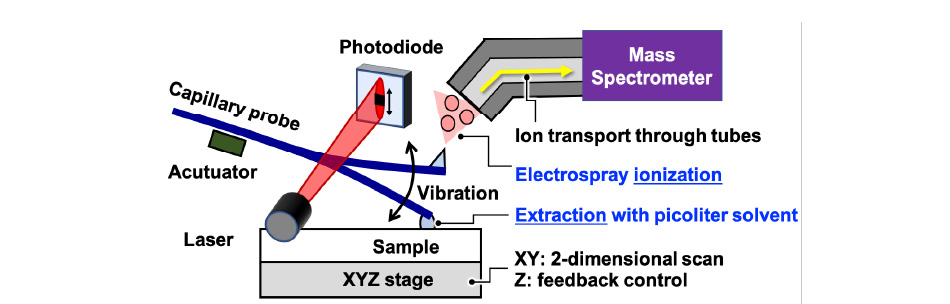
We have been developing tapping mode scanning probe electrospray ionization (t-SPESI) and mass spectrometric imaging of biological tissues. t-SPESI can perform extraction and soft ionization of multiple component of a sample in milliseconds by supplying charged pico-liquid through a vibrating capillary probe in the vertical direction.
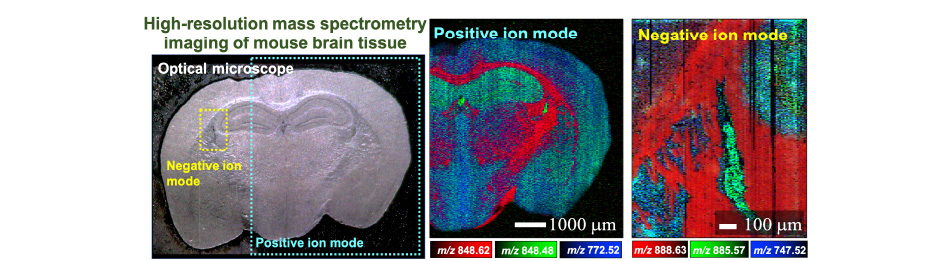
In order to stabilize the vibration of the capillary probe, we have developed techniques to measure the vibration of the probe and feedback control of the vibration amplitude, which enables us to visualize the distribution of lipids in uneven biological tissues with a spatial resolution of 6.5 micrometers.
In this study, we aim to understand the physics of energy dissipation due to the morphological change of the pico-liquid at the tip of the probe and the physical parameters required to stabilize the probe vibration and solvent flow.
Further development
Mass spectrometry imaging for understanding of disease states and its application to medical diagnosis
If we achieve a method to supply pico-liquid accurately to a micro-region, we will be able to clarify the distribution of components contained in an object with multiple components in a micro-region, such as a cell, and obtain new knowledge for unresolved research issues.
Applying mass spectrometry imaging by t-SPESI to human disease tissues, we will study the measurement of multi-dimensional chemical distribution information and feature extraction methods.
We aim to apply this technology to contribute to the elucidation of pathological status and to diagnostic technology that will lead to the realization of appropriate early treatment.
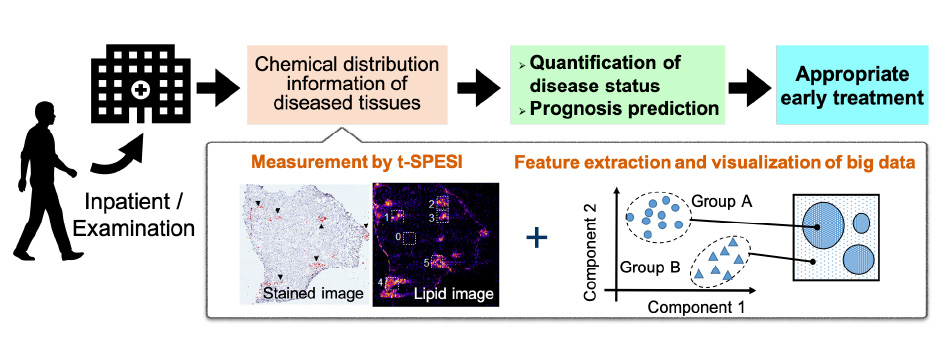
Through mass spectrometry imaging of diseased tissues and analysis of big data, we aim to quantify the state of disease and predict prognosis.